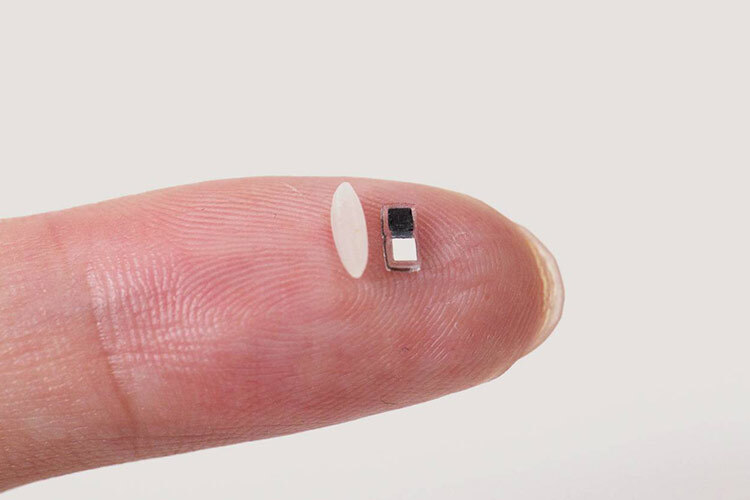
Researchers at Northwestern University in the United States have developed a pacemaker so small—just 1.8 mm wide and 3.5 mm long—that it can fit inside the needle of a syringe, making its application safe and minimally invasive.
Smaller than a grain of rice, this device is designed primarily for newborns with congenital heart defects, though it could also be used for larger hearts, according to a study published in Nature.
The pacemaker works in tandem with a portable, wireless device that sits on the patient’s chest to regulate stimulation. When it detects an irregular heartbeat, it sends out a light pulse strong enough to penetrate the skin and sternum, activating the pacemaker to restore the heart’s rhythm.
Once it reaches the end of its lifespan, the entire system naturally dissolves into the body’s fluids thanks to its bioresorbable design.
For Hearts Big and Small
The study, led by bioelectronics pioneer John A. Rogers and cardiologist Igor Efimov, demonstrated the device’s effectiveness in both small and large animal models, as well as in human donor hearts.
“We’ve developed what we believe is the world’s smallest pacemaker,” said Rogers, noting that the need for such devices in newborn patients is “critical.”
“Our primary motivation was children,” added Efimov, pointing out that roughly 1% of all children are born with congenital heart conditions.
The good news, according to Efimov, is that these children typically only need a pacemaker for a short period following surgery. After about seven days, most patients’ hearts heal on their own. “Now we can place it in a baby’s heart and stimulate it using a gentle, portable device,” he said—one that doesn’t require surgery to be removed.
Powered by the Body’s Own Fluids
To shrink the pacemaker’s size, researchers explored how to power it without the need for wires or receiver antennas.
Rather than using near-field communication (NFC), as they did in a previous prototype, the new device runs on a galvanic cell—essentially a battery that converts chemical energy into electricity.
Specifically, the pacemaker uses two different metals to deliver electrical pulses to the heart. When in contact with surrounding body fluids, the electrodes themselves turn into a functioning battery.
“When the pacemaker is implanted in the body, the surrounding biofluids act as the conductive electrolyte that electrically connects the two metal pads to form the battery,” Rogers explained.
Light-Activated Function
Engineers also realized that the human body is a surprisingly good conductor of light. They incorporated infrared light into the system to safely penetrate tissue and detect dips in heart rate.
When the external device picks up on an irregular heartbeat, it activates a light-emitting diode that flashes in sync with the rhythm of a healthy heart.
“The heart requires only minimal electrical stimulation,” Rogers said. “By shrinking the device, we drastically simplify implantation procedures, reduce trauma, and minimize patient risk,” he concluded.
A Reabsorbable Device
This work builds on a previous breakthrough by Rogers and Efimov, when they developed the first bioresorbable pacemaker. Their aim was to meet the needs of patients who required a temporary pacemaker while awaiting a permanent one—or to help restore normal heart rate during recovery.
Until recently, surgeons would sew electrodes directly onto the myocardium during surgery and connect them to an external pacemaker through the chest. Once the device was no longer needed, the electrodes were pulled out, often risking tears or tissue damage.
In response to this clinical challenge, they introduced a dissolvable pacemaker in Nature Biotechnology in 2021—a thin, flexible, and lightweight device that eliminated bulky batteries and wires altogether. (Via Sinc Agency)