By David Meléndez Martínez y Jorge Benavides
Venoms and poisons are substances attributed to the deaths of thousands of people around the world every year.1 Ironically, these secretions have been shown to be the source of bioactive compounds that enable the development of treatments to prevent and cure several diseases. In this article, we will explain what venoms and poisons are, what their biological role is, and how bioproducts with medical applications are being developed from them.
Since ancient times, animals have played a key role in society, participating in our diet, culture, religion, and medicine. Specifically, venomous and poisonous animals (see Glossary) (Figure 1) have inspired fear throughout human history. However, these very animals have also been the source of inspiration for developing treatments and medication that prevent the deaths of millions of people every year.
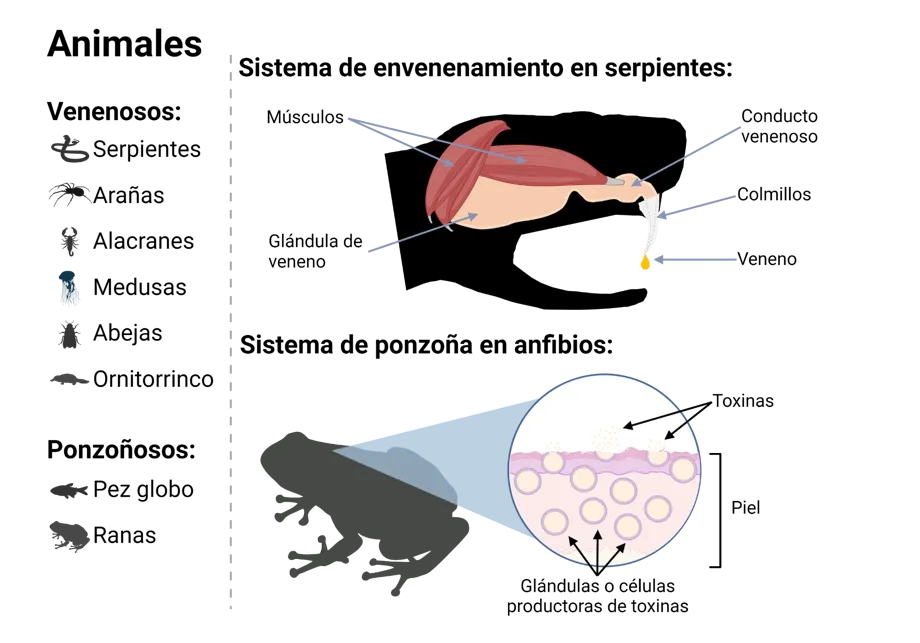
Animals:
Venomous: Venom delivery system in snakes:
Snakes Muscles, Venom Duct, Fangs, Venom, Venom Gland
Spiders
Scorpions
Jellyfish
Bees
Platypuses
Poisonous: Poison delivery system in amphibians:
Pufferfish Toxins, Skin, Toxin-producing glands or cells
Frogs
Different venoms and poisons consist of up to hundreds of molecules that we call toxins (see Glossary). It is important to remember that venoms and poisons are designed to fulfill an ecological role in animals as they use them for hunting their prey, predigesting it, or for defense against potential predators. Each toxin performs a specific activity that provides the venom with a targeted toxicity. For instance, when a rattlesnake hunts to feed, multiple toxins are released in its venom. Some cause muscular paralysis, while others predigest meat and fat. There are even aromatic toxins that help the snake to track its prey and hunt more efficiently.
As toxins are highly effective and each venom or poison has a large quantity of these, researchers tend to use them as a natural source for identifying bioactive compounds that can be applied in the development of new pharmaceuticals (see Glossary), thus utilizing the efficient molecular design that nature has perfected over the course of hundreds of millions of years.
From toxins to medicines: How they get to the pharmacy
Currently, many formulations with toxins as an active ingredient are sold as medication or are undergoing research to this end. The route for converting a toxin into a medicine requires multiple studies that enable researchers to identify, isolate, and prove the efficiency of potential active ingredients, as well as demonstrate that these bioactive formulations and compounds do not cause unwanted side effects (Figure 2).
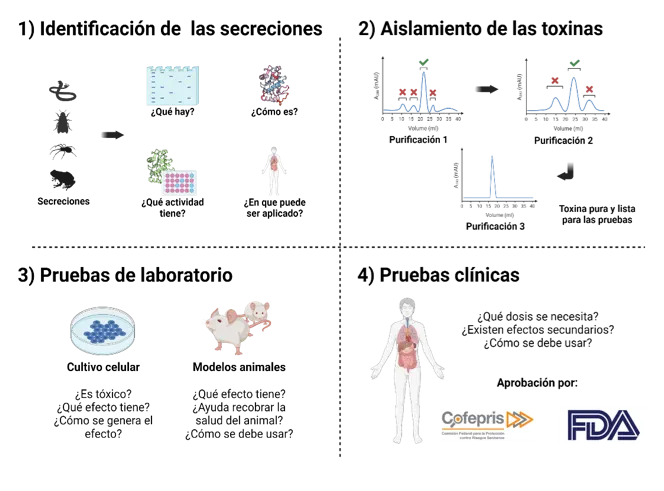
1) Identifying secretions
Secretions
What is there?
What is it like?
What activity does it have?
What can it be applied to?
2) Isolating toxins
Purification 1
Purification 2
Purification 3
Pure toxin ready for testing
3) Laboratory testing
Cell cultivation
Is it toxic?
What effect does it have?
How is the effect produced?
Animal models
What effect does it have?
Does it help the animal to recover?
How should it be used?
4) Clinical trials
What dose is needed?
Are there any side effects?
How should it be used?
This procedure is linked to an innovation management process based on identifying needs, areas of opportunity, and value-added solutions. To this end, it answers questions such as the following. Which disease requires a new medicine? Why do current medicines cause unwanted side effects? What is the disease’s biological background? Through which mechanism should the bioactive compound act? Although the process of developing a pharmaceutical from a venom or poison is complex, the sequence can be described in the following stages:
- Selecting which venoms or poisons to examine for the bioactive compounds of interest. These are chosen due to the qualities of the venom toxins; if the toxin required is already known, the venoms or poisons that contain it are selected.
- Identifying bioactive fractions from the partial fractioning of venoms and poisons. In vitro assays (such as enzyme or cell line assays) are used to do so in the laboratory, enabling identification of the fractions containing compounds with the specific activity required.
- Isolating, identifying, and characterizing bioactive compounds, while validating their individual activity through in vitro assays. The active fractions are separated into each of their compounds according to their chemical characteristics. Afterwards, the compounds that are most efficient at treating the disease will be identified.
- Characterizing and validating the therapeutic effect of bioactive compounds through in vivo studies (animal models). The protocols to be followed must be approved and validated by the corresponding bioethics committees. Animal models (mice or rats) are used to demonstrate therapeutic effects. Moreover, observations are made of unwanted effects that prevent this bioactive compound from being used as a pharmaceutical.
- Characterizing and validating the therapeutic effect in humans (clinical trials). In humans, demonstration is made of the therapeutic effect, the dose (see glossary) required by patients to be effective, and what possible side effects this bioactive compound could cause.
This sequence includes other stages related to the biotechnological production of bioactive compounds (to produce them at a large scale and in a standardized manner, without the need to exploit the original biological source) and to development of the formulation to stabilize these bioactive compounds and deliver them in a controlled and targeted manner. Once the compound (in its respective formulation) has proven to be effective and safe for use, approval for commercial use is required. This approval is granted by the Federal Commission for Protection against Health Risks (Cofepris) in Mexico and the Food and Drug Administration (FDA) in the United States.
Toxins in the pharmacy
Historically, the word “toxin” has been associated with harmful compounds. However, the only difference between a toxin and a pharmaceutical is the dose! A molecule in rationally defined doses can cause therapeutic effects, while it could be harmful or toxic in uncontrolled doses.
There are now pharmaceuticals inspired by toxins to treat multiple diseases or disorders. For instance, Exendin-4 from the venom of the Gila monster inspired Semaglutide for treating type 2 diabetes. Another example is the Ω-MVIIA peptide from the venom of sea snails, which inspired Ziconotide, a pharmaceutical used for treating chronic pain. Among the multiple medical applications that may exist for venoms and poisons, we at Tecnológico de Monterrey’s Institute for Obesity Research are striving to find toxins that can help us to prevent and combat obesity in children and adults, as well as associated diseases such as type 2 diabetes and metabolic syndrome.
Glosary
Dose: Amount of a pharmaceutical administered to someone to achieve a desired effect.
Pharmaceutical: A substance that serves to cure or prevent a disease. Synonyms: Drug, medication, medicine.
Poison: A toxic substance comprising one or more toxins, which harms the body through self-exposure or passive transfer without the need for any mechanism or release system. This is usually caused by ingestion, inhalation, or absorption through the skin.2 Moreover, certain poisonous animals do not produce their own toxins but obtain them from their surroundings.
Toxin: A substance produced by a living organism that can harm itself or another living organism. This is also known as a “biotoxin.” Note: Not to be confused with an environmental toxin or anthropogenic toxin.2
Venom: A toxic substance comprising one or more toxins, which needs to penetrate internal tissues via a wound to harm the body. The animals that produce this have a release system, which includes a group of cells that specialize in producing it, a duct, and a release mechanism, such as the fangs of a snake or the stinger of a bee or scorpion.2
Authors
David Meléndez-Martínez holds a PhD in Biotechnology. He is a postdoctoral researcher at the Bioengineering and Medical Device Unit of Tecnológico de Monterrey’s Institute for Obesity Research.
Jorge Benavides holds a PhD in Engineering Sciences with a specialization in Biotechnology. He is a research professor attached to the Bioengineering and Medical Device Unit of Tecnológico de Monterrey’s Institute for Obesity Research.
References
- Snakebite envenoming. Nat. Rev. Dis. Primers. 2017; 3, 17063. https://doi.org/10.1038/nrdp.2017.63
- Nelsen DR, Nisani Z, Cooper AM, et al. Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that employ them. Biol Rev. 2014;89(2):450-465. doi:10.1111/BRV.12062