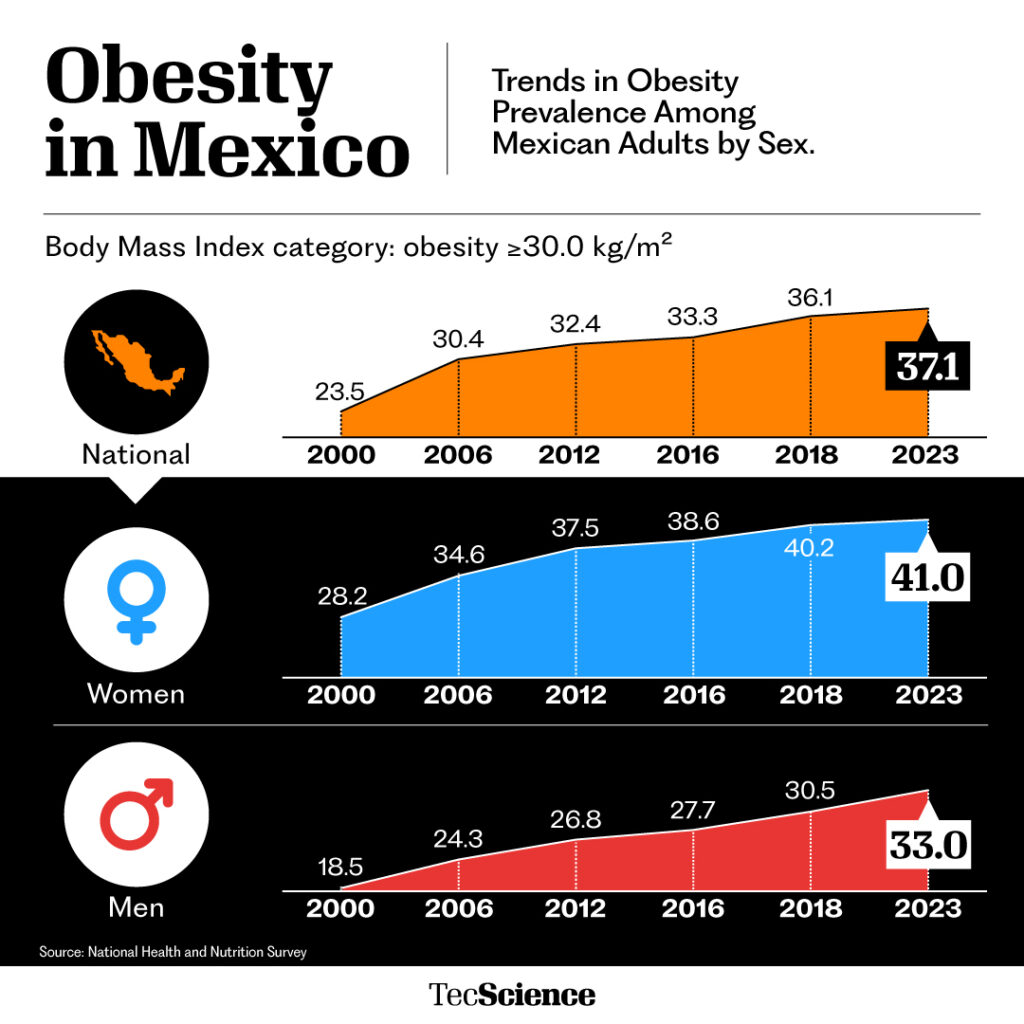
Mexico ranks among the top countries in the world for childhood overweight and obesity. According to the 2022 National Health and Nutrition Survey (Ensanut), 37.3% of children and 41.1% of adolescents are affected, roughly 15 million people in these age groups.
Obesity in school-age children and adolescents is linked to serious health problems, such as metabolic syndrome, fatty liver, hypertension, cardiovascular diseases, type 2 diabetes, and certain types of cancer. Additionally, it often leads to low self-esteem and social stigma.
With this in mind, researchers at the Institute for Obesity Research (IOR) at Tecnológico de Monterrey are working on a point-of-care testing device —similar to a pregnancy test—that could detect in minors a molecule associated with the development of overweight and obesity.
The project, called Lateral Flow Device for the Detection of the Protein Biomarker Leptin in Young Mexican Adults, involves collaboration with researchers from the University of Houston on technological development and validation, as well as with the National Institute of Nutrition and the Institute of Pediatrics in Mexico for clinical trials.
“It’s a platform that will allow us to detect a molecule or compound we’ve identified as a biomarker. Its presence or absence is directly linked to the disease. If it’s at certain levels, it can serve as an early warning sign,” explains Marco Antonio Rito, director of the IOR.
The Biomarker That Helps Detect Obesity Risk
As part of this project, the researchers reviewed more than 50 biomarkers to determine which one would be the most suitable for detection. Ultimately, they chose leptin, a biomarker with a well-established link to patient obesity.
Leptin is a hormone produced by fat cells, playing a key role in regulating hunger, the amount of fat stored in the body, and overall body weight. People who develop leptin resistance are at a higher risk of obesity.
“This is a pretty straightforward recommendation. The FDA and other agencies have confirmed that the presence or absence of this biomarker is associated with metabolic changes. If it weren’t, we’d face an additional challenge justifying why we selected it,” says Marco Rito in an interview with TecScience.
The test could trigger an alert by detecting this biomarker, allowing patients to consult with specialists and pursue a formal diagnosis.
While this technique is effective for the general population, Rito mentions that this project aims to focus on and validate it specifically for children. This presents an additional challenge in terms of clinical research, as testing must be conducted with minors.
The biomarker trials are built upon a previous project carried out by the research team, which aimed to detect biomarkers linked to type 2 diabetes, he adds.
Prototype Designed Like a Pregnancy Test
The device being developed by the researchers aims to provide an alternative for the early detection of metabolic changes that can eventually lead to overweight and obesity.
The biomarker is detected by analyzing a biological fluid sample, such as urine or blood. In individuals at risk of developing obesity, leptin can be found in higher concentrations.
For this study, the team has created a device with an internal “pad” in the shape of a rectangle, where the liquid sample flows laterally and the biomarker is applied. Rito explains that this lateral flow technology is a contribution from researchers at the University of Houston.
As the fluid moves across the surface, some biomarker molecules become trapped, making them visible. In other words, the user will see a control line, and if leptin is present, a second line will appear, indicating a positive result.
The researcher adds that while detecting these types of biomarkers already exists through the ELISA technique; patients currently have to visit a specialist and go to a laboratory. For instance, isolated populations require a cold chain to handle the samples.
“The principle behind developing this device is similar to other strategies. What sets us apart is the technology we are developing, which will allow us to meet certain criteria, such as speed, cost-effectiveness, and mass accessibility,” says the director.
Validating Technology in Children’s Population
The test development is being carried out by the Bioengineering and Medical Devices Unit at the IOR, which focuses on creating technologies for prevention and early detection.
Currently, the team is working on the clinical validation of the prototype with a controlled population, following the necessary protocols for minors. In addition to confirming its functionality, they also review the device’s adjustment parameters, such as its sensitivity and the time it takes to provide results.
Rito also points out that other studies are still needed in addition to validating the technology, such as determining how early this test could predict the risk of developing overweight or obesity.
“We expect to complete the clinical and validation phase in about six months. If all goes well, the next step—if we want to make a real impact—is to create a company that would enable mass production of the device, secure its approval and acceptance by Mexican regulations, and make it available to the population,” says Marco.
Did you find this story interesting? Would you like to publish it? Contact our content editor to learn more at marianaleonm@tec.mx