A study by microbiologists at the University of North Carolina (UNC) School of Medicine found that people with diabetes mellitus are more likely to develop infections caused by antibiotic-resistant bacteria.
The research, published in Science Advances, reveals how the microbial environment in people with diabetes promotes resistance mutations—and suggests new strategies to counteract this growing threat.
Staphylococcus aureus—a leading cause of infections and deaths linked to antibiotic resistance—is also the most common bacterial infection among diabetic patients. This chronic disease impairs blood sugar regulation and weakens the body’s ability to fight infections.
“We observed that antibiotic resistance emerges much faster in diabetic models than non-diabetic models of the disease,” said Brian Conlon, an associate professor in UNC’s Department of Microbiology and Immunology and co-author of the study published this week in Science Advances.
Conlon says, “this interaction between bacteria and diabetes may be a key factor in the rapid evolution and spread of antibiotic resistance we’re witnessing.”
How diabetes fuels resistance
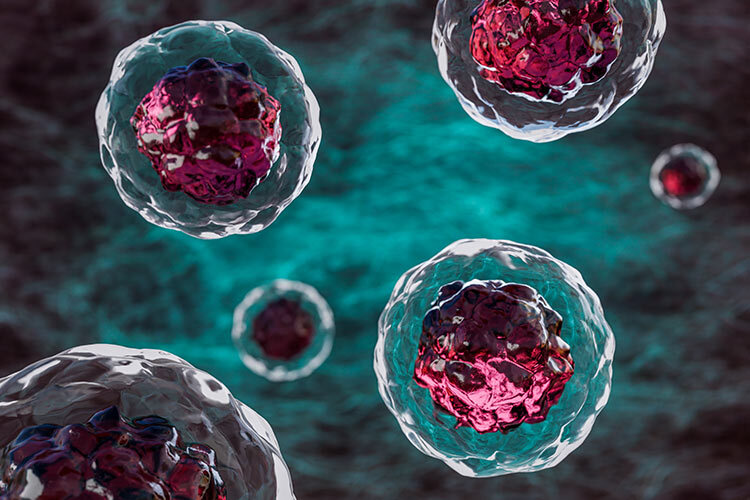
Diabetes leads to high glucose levels in the blood, which feeds Staphylococcus aureus and allows it to multiply faster. At the same time, a weakened immune system makes it harder to eliminate bacteria, giving them free rein to grow unchecked. The larger the bacterial population, the greater the chances of resistance mutations emerging.
Conlon and Lance Thurlow, professors of microbiology and immunology at UNC, used a mouse model to investigate how diabetes affects antibiotic effectiveness. They infected diabetic and non-diabetic mice with S. aureus and treated them with rifampin—an antibiotic known for quickly triggering resistance.
Diabetes and antibiotic resistance: What the study found
After just five days of treatment, the results were striking: rifampin had little effect in diabetic models. When researchers analyzed the samples, they found that the bacteria had developed resistance, with infected tissue harboring over 100 million resistant bacteria. In contrast, no resistant bacteria were detected in the non-diabetic mice.
The most surprising finding was how quickly resistance emerged: within just four days, resistant bacteria took over the infection in diabetic mice. However, when researchers administered insulin to lower blood glucose levels, they saw a sharp decline in bacterial growth and resistance mutations.
“Controlling blood sugar is crucial,” Conlon said. “When we gave insulin to the mice, their blood sugar levels normalized, and we didn’t see this rapid emergence of resistant bacteria.”
Next, Conlon and Thurlow plan to expand their research to human patients—both diabetic and non-diabetic—and investigate resistance in other bacteria, including Enterococcus faecalis, Pseudomonas aeruginosa, and Streptococcus pyogenes. They also aim to determine whether other vulnerable groups, such as chemotherapy patients or organ transplant recipients, face a heightened risk of antibiotic-resistant infections.
Antibiotic resistance remains a global concern, as resistant bacteria spread through the air, contaminated surfaces, and food. Preventing these infections is now more critical than ever. (AGENCIA SINC)