By María Isabela Avila Rodríguez, Mirna Lorena Sánchez, and Jorge Benavides
It is well known that diabetes mellitus is one of our country’s main health problems. However, did you know that 20-30% of diabetic patients are treated at least once for complications related to foot ulcers? Even more alarming is that if these complications are not treated in a timely and adequate manner, they can lead to the affected limb needing to be amputated.[1] [2]
In this article, we describe how the use of protease enzymes, such as bromelain, collagenase, and papain, form part of effective strategies for the treatment of diabetic foot ulcers.
Ordinary Reconstruction
For the sake of argument, let’s imagine that the skin is a factory of diverse scaffolding such as collagen, which we will call Healthy Skin Inc. In this factory, the different cells give orders to help maintain a healthy and resistant structure (Figure 1). When the factory process is interrupted, such as when we absent-mindedly cut our finger while opening a can of tuna, the cells send an urgent signal to stop the blood flow (called hemostasis) and cover the wound. An inflammation alert is also sent out, in which other groups of cells remove the mess created by the incident (debridement), degrade broken scaffolding, and eliminate invaders (bacteria) that could sneak into the factory through enzymatic tools (proteases).
Finally, the Healthy Skin Inc. factory resumes its activities, and cell groups such as fibroblasts and keratinocytes generate new scaffolding and build new pathways to close the wound so that everything works properly again.[3] The skin is then repaired.
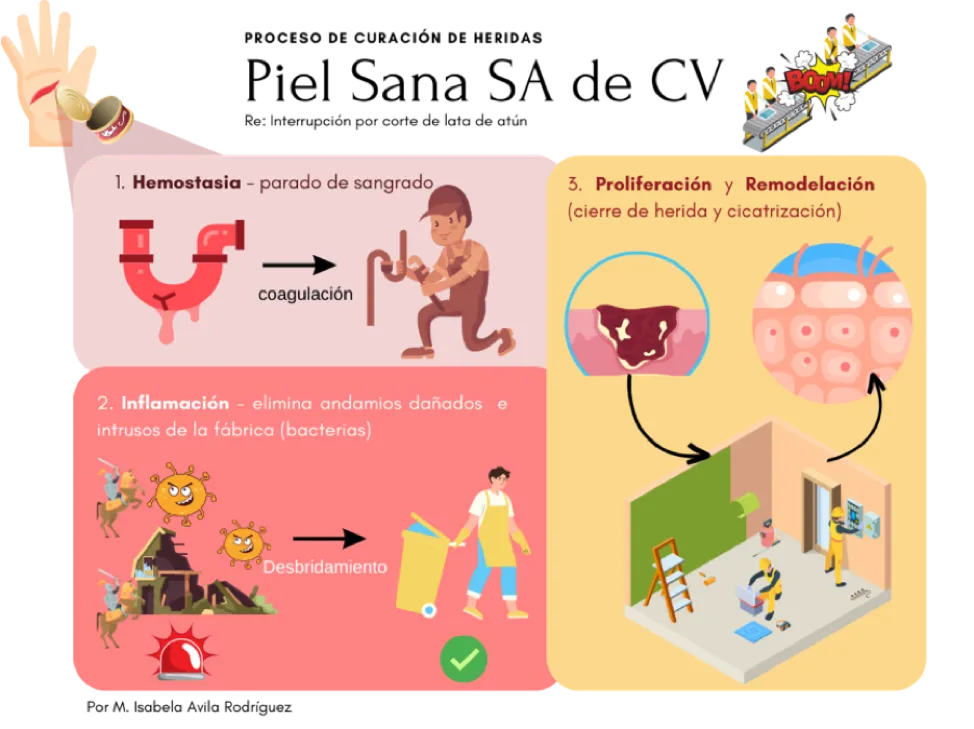
WOUND HEALING PROCESS. Healthy Skin Inc.
Re: Interruption due to a cut from a can of tuna
1. Hemostasis – stopping of bleeding
coagulation
2. Inflammation – eliminates damaged scaffolding and factory intruders (bacteria)
Debridement
3. Proliferation & Remodeling (closing of wound and healing)
By M. Isabela Avila Rodríguez
Insufficient Reconstruction
However, in patients with diabetic foot, the Healthy Skin Inc. factory suffers damage that does not allow for the proper closing of the wound, as in 1. Failure of the factory’s wound detection system due to damage to its main sensors, the nerves of the foot (diabetic neuropathy), and 2. Stagnation in the alarm phase (inflammation) due to excess and prolonged presence of sugar (hyperglycemia) in the area. This leads to damage and low oxygenation (hypoxia) in the circulatory system and abnormal clearance signals (hypercatabolism) of scaffolding that is not defective.[3] [4]
This in turn means that the factory’s repair system is unaware that there is a wound that needs to be repaired, and it therefore never heals the wound because it is stuck in an inflammatory phase. Over time, a covering called an eschar, composed of semi-degraded scaffolding and dead cells, is generated, which perpetuates the inflammatory state if not removed. Because of this accumulation, the body’s system of enzymatic tools is insufficient so that we need to rely on external tools to remove the eschar through a surgical, physical, or enzymatic method.
Non-invasive Intervention
As a first intervention, the patient is normally treated through surgical methods, with the hope that the wound will be able to emerge from stagnation and heal by eliminating the eschar. However, this is not always possible, and we then need a second method to resolve the stagnation.[5] In addition, surgical methods are not suitable for patients with coagulation problems,[3] so other specific and less invasive alternatives such as debridement using proteases (enzymes that break down proteins) are suitable since they act on the wound without requiring incision.
The enzyme treatment is applied to the surface of the wound. These enzymes function as molecular “sculptors,” which enter the wound and target the eschar’s partially degraded and accumulated scaffolding, thereby generating specific removal of the damaged tissue.
Since the technology requires only the ointment containing the protease enzymes and the application materials, it is considered cost-effective for chronic treatment without the need for an operating room when treating small wounds.
Biotechnological Advances
The enzymes that have been used in debridement procedures so far are extracted from plant sources, such as papaya or pineapple, and bacterial sources, such as Hathewaya histolytica. Part of the current challenges regarding the use of enzymes in debridement processes is finding proteases with a greater resemblance to those present in the wound healing process in humans to allow for more specific and compatible action with our skin.
Thanks to advances in the understanding of how proteases function in wound healing, the Bioengineering and Medical Devices unit of Tecnológico de Monterrey’s Institute for Obesity Research has been able to identify proteases in other animal sources similar to human proteases through computational methods. With modeling and structural comparison, it has been possible to identify enzymes that can be produced through biotechnological means in an economical and standardized way in yeasts such as Pichia pastoris. In turn, this will promote the development of formulations that will make it possible to treat a greater number of patients at a lower cost than with current treatments, following much less invasive processes and with quicker results. The research group led by Dr. Jorge Benavides also hopes to design and produce more specific and efficient active molecules through in silico –computer simulation– and protein engineering strategies, thus improving various aspects of current treatments for diseases that have a social impact.
The application of enzymatic debridement strategies in conjunction with timely diagnosis can prevent severe health complications in diabetic patients with foot ulcers, thereby significantly improving their quality of life.
Authors
María Isabela Avila Rodríguez is a Biotechnology doctoral candidate who has focused on the search for therapeutic molecules from animal sources for the closing of chronic wounds and severe burns through in silico and in vitro studies.
Mirna Lorena Sánchez is a member of the Molecular Pharmacology Laboratory of the National University of Quilmes, Argentina. Her research is focused on the development of organic and inorganic materials through electrospinning and electrodeposition to promote wound healing and achieve macrophage immunomodulation.
Jorge Benavides is a research professor at Tecnológico de Monterrey’s Institute for Obesity Research. His research is focused on the design of biotechnological processes and the development of bioproducts with diverse applications, including the prevention and treatment of diseases.
References
[1] A. V. A. Mariadoss, A. S. Sivakumar, C. H. Lee, y S. J. Kim, “Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical and molecular based treatment strategies via gene and nanotherapy,” Biomedicine & Pharmacotherapy, vol. 151, p. 113134, July 2022, doi: 10.1016/J.BIOPHA.2022.113134.
[2] G. García Rodríguez et al., “Informe Trimestral de Vigilancia Epidemiológica Hospitalaria Diabetes Mellitus Tipo 2 Corte al tercer trimestre 2022 Dirección de Vigilancia Epidemiológica de Enfermedades No Transmisibles,” Mexico, oct. 2022. Consulted: Jul. 17, 2023. [Online].
[3] M. I. Avila-Rodríguez, D. Meléndez-Martínez, C. Licona-Cassani, J. M. Aguilar-Yañez, J. Benavides, y M. L. Sánchez, “Practical context of enzymatic treatment for wound healing: A secreted protease approach (Review)”, Biomed Rep, Vol. 13, No. 1, p. 3, 2020, doi: 10.3892/BR.2020.1300.
[4] S. Jhamb, V. N. Vangaveti, y U. H. Malabu, “Genetic and molecular basis of diabetic foot ulcers: Clinical review,” J Tissue Viability, Vol. 25, No. 4, pp. 229–236, Nov. 2016, doi: 10.1016/J.JTV.2016.06.005.
[5] J. Paredes y J. Lantis, “Permissive maintenance debridement — the role of enzymatic debridement in chronic wound care,” Wounds International, Vol. 8, No. 2, pp. 7–13, jun. 2017, [Online].