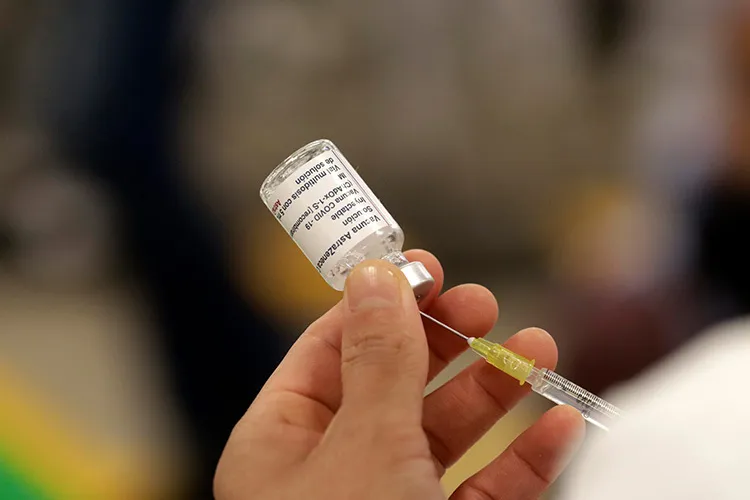
In October 2021, the Mexican government was relieved to announce the arrival of the largest shipment ever of the Covid-19 vaccine.
The shipment contained a batch of 3,412,900 doses of the vaccine manufactured by AstraZeneca later to be known as Vaxzevria. This announcement went on to reveal that the vaccines had been donated by the United States and subsequently sent to eighteen Mexican states.
It also noted that “with this shipment, 54,829,500 AstraZeneca doses (in total) have been made available to our country”.
According to the data, AstraZeneca (Vaxzevria) vaccines accounted for forty percent of all vaccines brought into the country.
This is the very same vaccine that has just been taken off the market following the legally documented revelation in a court case in the United Kingdom that it caused thrombosis and low blood platelet levels.
By March 2022, although Mexico had already acquired more than 112 million doses, the vaccine did not obtain standard marketing authorization in Europe until October 2022.
The Adenovirus that Primed the Body Against Covid-19
A year after that shipment in 2022, the European Medicines Agency wrote a report on the characteristics of Vaxzevria explaining, for instance, that the remedy acts by preparing the body to defend itself against Covid19.
To quote the report, “It is made up of another virus (adenovirus) that has been modified to contain the gene for making the SARS-CoV-2 spike protein. This is a protein on the surface of the SARS-CoV-2 virus which the virus needs to enter the body’s cells.”
When the protein has been administered, the body’s immune system recognizes it as foreign and produces antibodies while the cells prepare to attack it.
If someone then becomes infected with Covid-19, the body recognizes it and is ready to defend itself. According to the report, the adenovirus cannot reproduce itself and did not bring on the disease.
Negative Side Effects
However, the European agency’s report warned about the risk of side effects such as headaches, fever, and nausea. Moreover, it may cause low blood platelet levels in one out of every ten patients, resulting in dizziness, fatigue, and even gum and gastrointestinal bleeding.
“Thrombosis (formation of blood clots in the blood vessels) in combination with thrombocytopenia (thrombosis with thrombocytopenia syndrome, TTS) and Guillain-Barré syndrome (a neurological disorder in which the body’s immune system damages nerve cells) may affect up to 1 in 10,000 people,” the report explained.
This notwithstanding, the European agency accepted the vaccine because of the pressing need to combat the pandemic and because testing had shown sixty percent effectiveness against Covid-19.
For its part, the European Commission withdrew marketing authorization for the AstraZeneca vaccine, at least in the European Union, as of March 27, 2024.
The request was made by the company’s marketing area, which gave notice of the decision to discontinue the sale of the vaccine.
It was widely reported that the company is facing collective legal action in Britain due to negative side effects on health that had previously been identified by scientists and the European Medicines Agency.
In 2021, after the administration of 37 million doses in the UK, June Raine, the Chief Executive of the UK Medicines and Healthcare products Regulatory Agency, announced that “there is no risk-free effective medicine or vaccine”.
Similarly, a report released on March 31, 2021, reported two cases of thrombosis with thrombocytopenia (TST) caused by the Pfizer vaccine after 14.5 million doses had been administered in the country, of which 3.5 million were second doses.
Meanwhile, eight million doses of the Jenssen vaccine were administered in the United States prior to April 2021 with seventeen recorded incidences of thrombosis with thrombocytopenia (TST) as reported in a study done in Catalonia, Spain called Thrombosis and Thrombocytopenia after Vaccination against and Infection with SARS-CoV-2 and published in Nature Communications.
What Other Studies Say
Research published in Nature in November 2022 reported that, among the 5.6 million people vaccinated against Covid-19 in the United Kingdom, thrombosis, thrombocytopenia and thrombosis with thrombocytopenia were very rare events indeed.
The study clarifies that post-vaccination thrombocytopenia had previously been reported after inoculation with other vaccines, such as those for influenza, measles, mumps and rubella, and hepatitis B.
However, the incidence of thromboembolism after vaccination was acknowledged to be 1.1 times higher than expected in the general population and more than 7 times the expected rate among patients infected with Covid-19.
Moreover, it was reiterated that Covid-19 infection prior to any Covid vaccination was associated with increased risk of thrombocytopenia, arterial thromboembolism and thrombosis with thrombocytopenia, according to a study headed up by Edward Burn of the Jordi Gol i Gurina University Institute for Primary Health Care Research Foundation in Barcelona and Xintong Li of the University of Oxford Centre for Statistics in Medicine.
“These results underscore the relative safety of the vaccines compared with the numerous deleterious effects of Covid-19 infection for people who have not been inoculated,” the study stated.
Did you find this story interesting? Would you like to publish it? Contact our content editor to learn more at marianaleonm@tec.mx