By Helen Yarimet Lorenzo-Anota, Andrés Galindo-Padrón, Jorge L. Cholula-Díaz and Omar Lozano
Cardiovascular diseases are the leading cause of death in Mexico, according to 2025 figures from INEGI [1]. These conditions involve problems related to the heart and blood vessels [2].
The use of metallic nanoparticles in cardiovascular disease marks a scientific and technological breakthrough, enabling researchers to gain a deeper understanding of these conditions and explore new treatment possibilities.
Recently, metallic nanoparticles have been employed to detect biomarkers—biological indicators of disease— like the prostate-specific antigen (PSA), a blood protein that can indicate the presence of prostate cancer. They are also employed as diagnostic tools to visualize internal tissue structures, for example, to detect inflammation in blood vessels [3].
Their medical use is possible because metals exhibit unique physical, chemical, and biological properties at the nanoscale (10⁻⁹ m) [4]. For instance, gold, typically a yellow precious metal, can appear in shades ranging from red-purple to blue at the nanoscale, depending on particle size and shape.
This simple color transformation reflects changes in their properties, expanding their applications as optical sensors, markers, or therapeutic tools.
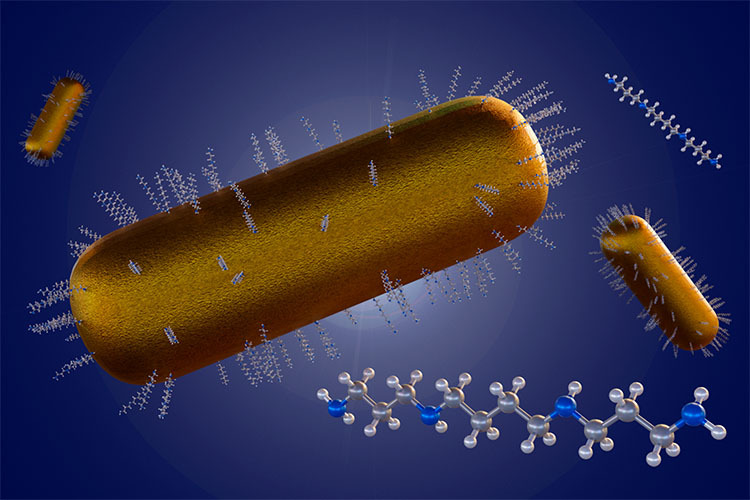
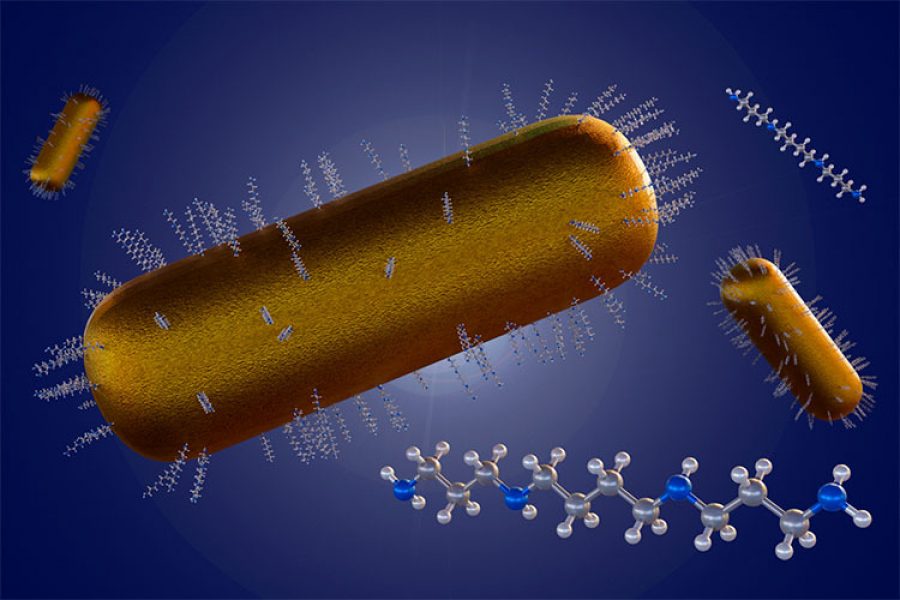
Moreover, it is possible to create bimetallic nanoparticles composed of two different metals [5]. These combinations can offer enhanced or entirely new properties compared to single-metal (monometallic) nanoparticles.
However, the effects of these nanoparticles are still under active investigation.
Bimetallic nanoparticles: purpose and potential
The scientific paper Study on the regulated cell death of hypertrophic H9C2 cells induced by Au:Ag nanoparticles aimed to evaluate the effects of bimetallic gold-silver nanoparticles on cardiac cells under conditions mimicking cardiovascular disease.
Bimetallic gold-silver nanoparticles were produced in different ratios using a green synthesis method—an eco-friendly process that uses natural materials to fabricate the particles. Starch was used as a dual-function agent, serving as both a reducer and stabilizer [6]. Thanks to the starch, potential negative effects on cardiovascular cells were minimized, and the nanoparticles remained stable.
The resulting nanoparticles exhibited promising biological potential, with particle sizes ranging from 13 to 30 nm, spheroidal shapes, and prolonged stability in colloidal form for over a month.
To explore potential applications in cardiovascular disease, researchers studied healthy heart cells and cells with hypertrophy—cells that have increased in size, reducing the heart’s efficiency.
These bimetallic nanoparticles were also tested on macrophages, cells that play a crucial role in inflammation progression.
The study found that silver nanoparticles are more cytotoxic than gold, and combining gold with silver can reduce the cytotoxicity of silver nanoparticles.
Cardiac cells with hypertrophy were more sensitive to bimetallic nanoparticles, particularly those with high silver content (↑silver = ↓cell viability). Gold and silver nanoparticles increased the production of reactive oxygen species, causing mitochondrial damage and cell death.
Interestingly, macrophages were not affected by exposure to gold and silver nanoparticles, even under inflammation induced by fatty acids.
Findings and applicability
While these results support the potential use of bimetallic gold-silver nanoparticles in cardiovascular disease, it is important to note the heightened susceptibility of hypertrophic cells compared to healthy ones.
In other words, interventions involving these nanoparticles—whether for diagnostic or therapeutic purposes—should be approached with caution in patients with cardiovascular conditions.
The clinical application of bimetallic nanoparticles in cardiovascular medicine still faces numerous challenges, particularly in the use of animal models to assess biocompatibility, organ toxicity, and disease-specific effects.
This research lays the groundwork for future studies, including 3D cell cultures and animal experiments, where bimetallic nanoparticles could serve as key tools in biomedical applications, such as drug delivery systems and biosensors.
.
References
- Estadística de Defunciones Registradas (EDR) de enero a junio de 2024. (2025, January 22). INEGI. Retrieved June 24, 2025, from
- What is Cardiovascular Disease? (2024, January 10). American Heart Association. Retrieved June 24, 2025, from
- Liu, Y., Lin, Z., Wang, Y., Chen, L., Wang, Y., & Luo, C. (2024). Nanotechnology in inflammation: cutting-edge advances in diagnostics, therapeutics and theranostics. Theranostics, 14(6), 2490–2525.
- Joudeh, N., & Linke, D. (2022). Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. Journal of Nanobiotechnology, 20(1).
- Toshima, N., & Yonezawa, T. (1998). Bimetallic nanoparticles—novel materials for chemical and physical applications. New Journal of Chemistry, 22(11), 1179–1201.
- Galindo-Padrón, A., Lorenzo-Anota, H., Rueda-Munguía, M., García-Carrasco, A., López, M. G., Vázquez-Garza, E., Campos-González, E., Lozano, O., & Cholula-Díaz, J. (2025). Study on the regulated cell death of hypertrophic H9C2 cells induced by Au:Ag nanoparticles. International Journal of Nanomedicine, 20, 1491–1507.
.
Authors
Helen Yarimet Lorenzo Anota. PhD in Immunobiology from UANL. She is currently a postdoctoral researcher at the Institute for Obesity Research, Bioengineering and Medical Devices Unit, Tecnológico de Monterrey. Her research focuses on developing systems for the efficient delivery of molecules with anti-obesogenic activity in in vitro models. Since 2023, she has been a Level I member of the National System of Researchers (SNI, México).
Andrés Galindo-Padrón. He earned his degree in Nanotechnology Engineering and Chemical Sciences, with a concentration in Neuroscience, from Tecnológico de Monterrey in 2023. Since then, he has been part of the research laboratory at the School of Medicine and Health Sciences, Hospital Zambrano Hellion.His work focuses on the synthesis and nanotoxicology of metallic nanomaterials, particularly precious metals.
Jorge L. Cholula-Díaz. He received his PhD in Natural Sciences with an emphasis on Chemistry and Nanotechnology from the University of Leipzig (Germany) in 2013. Since 2015, he has been a faculty researcher at the School of Engineering and Sciences (EIC), Tecnológico de Monterrey, Mexico. His scientific research focuses on the sustainable synthesis of various nanomaterials, including noble metals and metal oxides, in colloidal form or as thin films, with applications in nanomedicine, electro- and photocatalysis. He is a Level I member of the National System of Researchers (SNI) and a member of the Chemical Society of Mexico (SQM).
Omar Lozano. Faculty researcher at the Institute for Obesity Research and the School of Medicine and Health Sciences, Tecnológico de Monterrey. His research focuses on developing advanced, safe, and effective materials for delivering therapeutic and diagnostic agents to treat cardiometabolic diseases. He is the author of 46 scientific and popular science publications (h-index: 20), 4 patents, and 2 book chapters. He is a Level II member of the National System of Researchers (SNI, Mexico) and a Chargé de Recherche (Belgium).