Researchers from the Massachusetts Institute of Technology (MIT) have discovered the existence of “neutronic molecules.” We explain the importance of this discovery to the world of physics and what its applications are.
Firstly, the research entitled “μeV-Deep Neutron Bound States in Nanocrystals” was published in March in the journal ACS Nano. It was written by MIT graduate students Hao Tang and Guoqing Wang and professors Ju Li and Paola Cappellaro from the Department of Nuclear Science and Engineering at the same institution.
As an article published on the MIT website explains, “neutrons are subatomic particles that have no electric charge, unlike protons and electrons. That means that while the electromagnetic force is responsible for most of the interactions between radiation and materials, neutrons are essentially immune to that force.”
“Instead, neutrons are held together inside an atom’s nucleus solely by something called the strong force, one of the four fundamental forces of nature.”
Although it may sound redundant, MIT adds that “as its name implies, the force is indeed very strong, but only at very close range: it drops off so rapidly as to be negligible beyond 1/10,000 the size of an atom.”
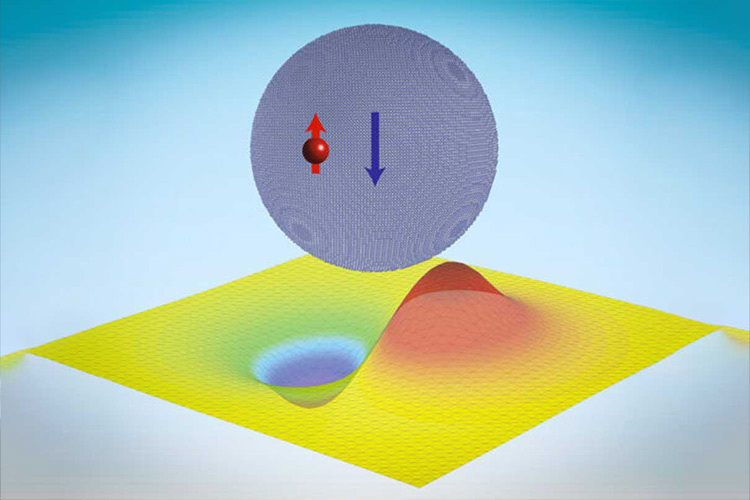
What the research led by Li and Cappellaro has discovered is “that neutrons can actually be made to cling to particles called quantum dots, which are made up of tens of thousands of atomic nuclei, held there just by the strong force.”
What Does The Discovery Of “Neutronic Molecules” Consist Of?
“Neutronic molecules” are described by the researchers as “a new state” that offers a way to “probe the mysterious inner workings of materials and unlock new frontiers in quantum information processing.”
In an interview published on the MIT website, Ju Li said: “we were surprised that the neutrons can be trapped by the materials and that nobody had talked about this before, among the experts we had checked with.”
He added that the force holding the nuclei of atoms together “is very small, but it’s very intense… What’s interesting is we’ve got these thousands of nuclei in this neutronic quantum dot, and that’s able to stabilize these bound states, which have much more diffuse wavefunctions at tens of nanometers [billionths of a meter].”
Li says that these neutronic bound states in a quantum dot “are actually quite akin to Thomson’s plum pudding model of an atom, after his discovery of the electron.”
Neutrons are widely used to probe material properties using a method called neutron scattering, in which a beam of neutrons is focused on a sample, and the neutrons that bounce off the material’s atoms can be detected to reveal the material’s internal structure and dynamics.
However, until this new publication, nobody thought that these neutrons might actually stick to the materials they were probing.
What Are “Neutronic Molecules”?
Paola Cappellaro remarks on the MIT website that “in conventional quantum dots, an electron is trapped by the electromagnetic potential created by a macroscopic number of atoms, thus its wavefunction extends to about 10 nanometers, much larger than a typical atomic radius.”
“Similarly, in these nucleonic quantum dots, a single neutron can be trapped by a nanocrystal, with a size well beyond the range of the nuclear force, and display similar quantized energies.” While these energy jumps give quantum dots their colors, the neutronic quantum dots could be used for storing quantum information.
The researchers mention that the “artificial atoms” made up of assemblages of atoms that share properties and can behave in many ways like a single atom have been used to probe many properties of real atoms.
Similarly, these artificial molecules provide “an interesting model system” that might be used to study “quantum mechanical problems that one can think about, such as whether these neutronic molecules will have a shell structure that mimics the electron shell structure of atoms.”
What Are The Applications Of “Neutronic Molecules”?
For these experts, one possible application is that “maybe we can precisely control the neutron state. By changing the way the quantum dot oscillates, maybe we can shoot the neutron off in a particular direction.”
Neutrons are powerful tools for such things as triggering both fission and fusion reactions, but so far it has been difficult to control individual neutrons. These new bound states could provide much greater degrees of control over individual neutrons, which could play a role in the development of new quantum information systems.
It’s important to mention that although quantum information processing is currently based on electromagnetism, Ju Li says that the “neutronic molecule” could serve as a mediator between the nuclear spins of separate nuclei and “this nuclear spin is a property that is already being used as a basic storage unit, or qubit, in developing quantum computer systems.”
Applications could also include medicine; the researchers say that imaging can be done using neutral activation analysis. “Neutron imaging complements X-ray imaging because neutrons are much more strongly interacting with light elements.”
“It can also be used for materials analysis, which can provide information not only about elemental composition but even about the different isotopes of those elements. A lot of the chemical imaging and spectroscopy doesn’t tell us about the isotopes, whereas the neutron-based method could do so”.
Another of the applications for this discovery could be related to atomic energy, according to the site Interesting Engineering: “This breakthrough could offer unprecedented control over individual neutrons, leading to advancements in nuclear reactions (fission and fusion) for safer, more efficient energy production.”