By Dr. Jorge E. Valdez-García, Dr. Denise Loya García, Dr. Judith Zavala Arcos, MPSS David E. Rodríguez-Fuentes, all members of the Advanced Therapies in Visual Sciences Research Group
The human body is capable of responding to disease or injury by itself. However, advances in science have sped up the process of regeneration through the development of various treatments to restore function and health to damaged tissues and organs. This is where advanced therapies play their part. The concept of advanced therapy refers to the combination of new medical products that use gene or cell therapy, tissue-engineered products, and a combination of both. Examples of these include the use of cells for biomedical devices, for treatment of diseases for which there is still no cure, or for which only symptomatic treatments exist.[1]
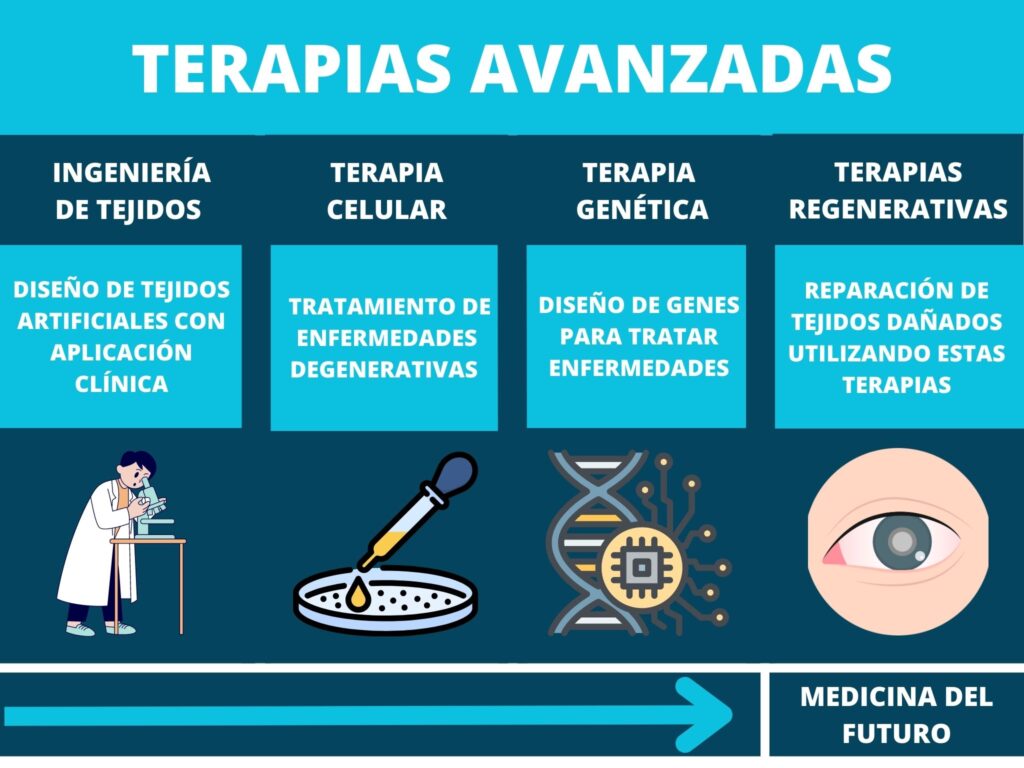
Advanced therapies provide a unique multidisciplinary platform for research into the next generation of treatments, such as targeted drug delivery, tissue regeneration, and personalized medicines and compounds for simultaneous diagnostic and therapeutic applications.
Advanced therapies are products for human use with active therapeutic principles based on at least one of the following procedures:[2]
- In vivo therapies using nucleic acids or genetically modified organisms (viruses, bacteria, or fungi).
- Ex vivo therapies that genetically modify cells or edit the human genome.
- Cells that have been engineered (for example, grown and activated from different molecules) for homologous use.
- Cells that are intended to be used for a different basic function in the patient from the donor (for non-homologous use).
- A combination of the above with devices such as biomaterials or instruments for the diagnosis, monitoring, or treatment of a disease.
Advanced therapies for ophthalmology
For several years, there have been lines of research on advanced therapies in ophthalmology, whose aim is to design specific treatments. One of the first therapies to be studied is based on stem cells [3].
Corneal stem cells are found in the basal layer of the limbus (located in the transition zone between the cornea and the sclera) and are responsible for proper corneal healing and repair. There are various corneal pathologies in which the corneal limbus is compromised. Among the most common are corneal burns, after eye surgery, or even as a side effect to the overuse and/or improper use of contact lenses. In order to restore the function of the corneal epithelium and the ocular surface, proper treatment would be a limbal stem cell transplantation.[4]
Likewise, the treatment used to counteract unilateral limbal stem cell deficiency for the past 30 years continues to involve transplantation of limbal tissue from the contralateral eye. However, in bilateral cases, transplantation of related living tissue or cadaveric tissue is necessary. The first studies on ex vivo cultured limbal epithelial cell grafts began in 1997. In 2014, a product with these characteristics was approved for sale in Europe.[5]
According to the World Health Organization (WHO), a total of 2 billion people were estimated to have vision impairment or blindness in 2019, and at least half of them had a type of vision impairment that could have been prevented or had yet to be treated.[6] Cataracts continue to be the most common cause of blindness to date, followed by corneal blindness. The cornea is the transparent tissue at the front of the eye that allows light rays to enter and focus correctly onto the back of the eye, i.e., the retina. The most common causes of corneal blindness include severe infections, trauma, vitamin deficiency, and corneal scarring. The presentation and frequency of these vary according to the geographical area and population studied.[7]
Corneal blindness due to endothelial dysfunction is considered one of the main reasons for corneal transplantation. However, there is a significant shortage of tissue available in our country. In Mexico, there are over 4,000 people on the waiting list to receive a cornea transplant, and waiting times can range from 9 months to 2 years [8]. This is why there is a great deal of interest in the development of corneal endothelial cells through cell therapy and tissue engineering. The aim is to create cellular tissue in a laboratory that is capable of replacing organs and tissues which are damaged and have lost their function, whether due to disease, injury, or genetic alteration. To develop this, cells, growth factors, and a suitable culture medium are required for proliferation, as well as a biocompatible scaffold for transplantation.
Over the past 10 years, Tec de Monterrey’s Advanced Therapies in Visual Sciences research group has devoted itself to the cultivation, expansion, and growth of corneal endothelial cells on a biocompatible and functional scaffold, with the aim of creating a therapeutic option available for corneal diseases and providing a solution to the tissue shortage, thus contributing to improving our patients’ quality of life.[9-17]
The future of ophthalmological treatments
Advanced therapy development has accelerated over recent years, which represents significant benefits for improving the quality of life of patients with ophthalmological diseases.
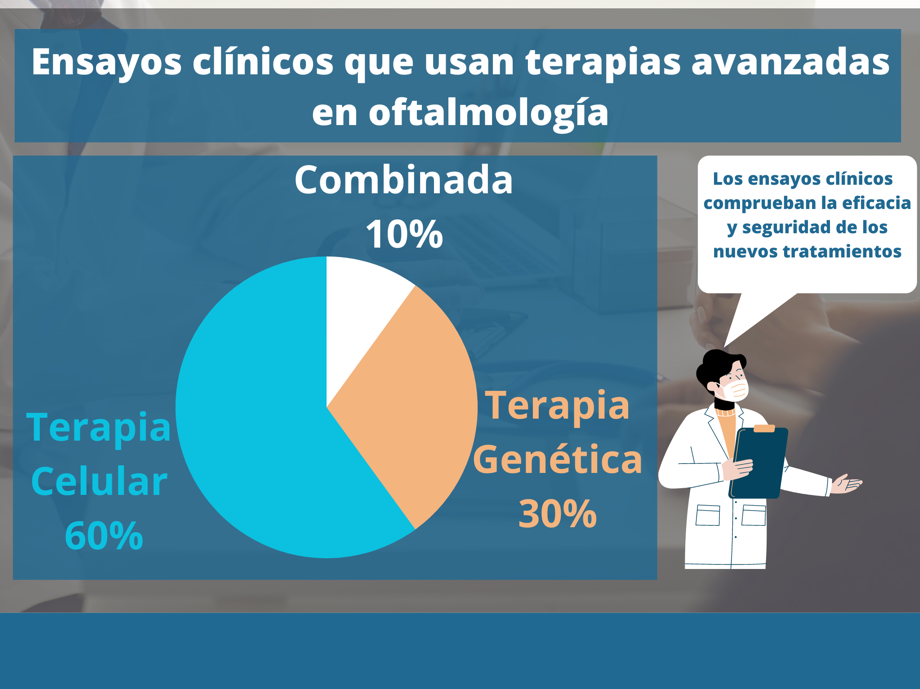
Currently, around 60% of ongoing clinical trials involving advanced therapies for the treatment of ocular diseases are focused on cell therapy for retinal and optic nerve diseases, ocular surface diseases, uveal melanoma, and glaucoma. In second place, (around 30% of trials) clinical research is focused on gene therapy for the treatment of diseases such as Leber congenital amaurosis, macular diseases, and retinitis pigmentosa. The smallest proportion (around 10%) is made up of combined therapies focused on retinitis pigmentosa, glaucoma, and macular degeneration.[18-19]
Regenerative medicine has played a very important role in the development of promising new therapies for the treatment of ophthalmological diseases.
Advanced therapies make us change the way we think about how to treat pathologies. Imagine a world where diseases could be cured with a single cell, tissue, or organ created from your own cells… This future is closer than we think, and it is called personalized medicine.
Bibliography:
- Magrelli FM, Merra A, Pellegrini G. Surgery Versus ATMPs: An Example From Ophthalmology. Front Bioeng Biotechnol. 2020;8:440. Published 2020 Jun 10. doi:10.3389/fbioe.2020.00440
- Hanna E, Rémuzat C, Auquier P, Toumi M. Advanced therapy medicinal products: current and future perspectives. J Mark Access Health Policy. 2016;4:10.3402/jmahp.v4.31036. Published 2016 Apr 25. doi:10.3402/jmahp.v4.31036
- Figueiredo FC, Glanville JM, Arber M, et al. A systematic review of cellular therapies for the treatment of limbal stem cell deficiency affecting one or both eyes. Ocul Surf. 2021;20:48-61. doi:10.1016/j.jtos.2020.12.008
- de Araujo, A. L. (2015). Corneal Stem Cells and tissue engineering: Current advances and future perspectives. World Journal of Stem Cells, 7(5), 806. https://doi.org/10.4252/wjsc.v7.i5.806
- Pellegrini G, Ardigò D, Milazzo G, et al. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl Med. 2018;7(1):146-154. doi:10.1002/sctm.17-0003
- OMS. (2019). La Oms Presenta el primer informe Mundial sobre la visión. World Health Organization. Retrieved March 21, 2023, from https://www.who.int/es/news/item/08-10-2019-who-launches-first-world-report-on-vision#:~:text=Al%20menos%202200%20millones%20de,a%C3%BAn%20no%20han%20sido%20tratados
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214-221.
- CENATRA. (2022). 2do informe trimestral 2022 sobre donación y trasplante. Documento Sin Título. Retrieved March 21, 2023, from https://cenatra.salud.gob.mx/transparencia/trasplante_estadisticas.html
- ZO-1 and Na/K ATPase are expressed in a human-engineered corneal endothelium. Maria Dolores Montalvo Parra, Judith Zavala, Manuel Chacon, Natalia Vazquez, Alvaro Meana, Jesus Merayo-Lloves, Jorge E Valdez. Investigative Ophthalmology & Visual Science 61 (7), 1204-1204, 2020
- 3D epiflourescence analysis of a bioengineered CEC scaffold. Betsabe Hernández-Sedas, Maria Dolores Montalvo Parra, Cesar E Calzada-Rodriguez, Judith Zavala, Jorge E Valdez. Investigative Ophthalmology & Visual Science 61 (7), 1202-1202, 2020.
- Stem cell reprogramming towards corneal endothelial cell using CRISPR-dCAS9. Guillermo Isaac Guerrero Ramirez, Victor Treviño, Jorge E Valdez, Judith Zavala, Emmanuel Martinez-Ledesma. Investigative Ophthalmology & Visual Science 60 (9), 4955-4955, 2019.
- Characterization of a collagen-based engineered corneal endothelium. Maria Dolores Montalvo Parra, Judith Zavala, Wendy Ortega-Lara, Jorge E Valdez-Garcia. Investigative Ophthalmology & Visual Science 60 (9), 4119-4119, 2019.
- Novel mutations associated with keratoconus found by a bioinformatic approach. Daniela Gomez-Elizondo, Mariana Lopez-Martinez, Judith Zavala, Jorge E Valdez-García, Victor Treviño. Investigative Ophthalmology & Visual Science 60 (9), 387-387, 2019.
- Expression profiling of stem cell for differentiation into corneal endothelium. Alejandro Tamez, Guillermo Guerrero-Ramirez, Victor Treviño, Jezreel Pantaleon-Garcia, Judith Zavala, Jorge E Valdez. Investigative Ophthalmology & Visual Science 58 (8), 1466-1466, 2017.
- Differential gene expression pattern between corneal endothelium cells and dental pulp stem cells. Julio C Hernandez, Pedro Romero, Victor Treviño, Judith Zavala, Jorge E Valdez. Investigative Ophthalmology & Visual Science 56 (7), 3461-3461, 2015.
- Gene expression analysis between retinoblastoma with exophytic and endophytic growth patterns. Preliminary analysis. Carlos-Alberto Rodríguez-Barrientos, Judith Zavala, Jorge Valdez, Victor Treviño, Juan Martinez-Ledesma. Investigative Ophthalmology & Visual Science 54 (15), 1173-1173, 2013
- Montalvo-Parra, M.D.; Ortega-Lara, W.; Loya-García, D.; Bustamante-Arias, A.; Guerrero-Ramírez, G.-I.; Calzada-Rodríguez, C.E.; Torres-Guerrero, G.F.; Hernández-Sedas, B.; Cárdenas-Rodríguez, I.T.; Guevara-Quintanilla, S.E.; Salán-Gomez, M.; Hernández-Delgado, M.Á.; Garza-González, S.; Gamboa-Quintanilla, M.G.; Villagómez-Valdez, L.G.; Zavala, J.; Valdez-García, J.E. Customizable Collagen Vitrigel Membranes and Preliminary Results in Corneal Engineering. Polymers 2022, 14, 3556. https://doi.org/10.3390/polym14173556
- López-Paniagua M, de la Mata A, Galindo S, Blázquez F, Calonge M, Nieto-Miguel T. Advanced Therapy Medicinal Products for the Eye: Definitions and Regulatory Framework. Pharmaceutics. 2021 Mar 6;13(3):347. doi: 10.3390/pharmaceutics13030347. PMID: 33800934; PMCID: PMC8000705.
- Iglesias-Lopez, C., Agustí, A., Obach, M. and Vallano, A., 2019. Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States. Frontiers in Pharmacology, 10.